OROPALLO CASE SERIES
The study showed how PuraPly® AM reduced wound size in chronic wounds that failed ~2 years of prior therapies.1
OBJECTIVE
Prospective case series to assess the ability of PuraPly AM to manage bioburden, support granulation tissue formation, and support wound closure over 12 weeks in chronic, nonhealing wounds.
STUDY DESIGN
- A single-center, prospective case series (NCT03070925)
- Wounds were cleaned and prepared with sharp or mechanical debridement at initial visit
- PuraPly AM was applied at week 0 and then every 1-3 weeks thereafter
- At each visit, wounds were assessed for signs of infection, wound area and depth, and achievement of closure
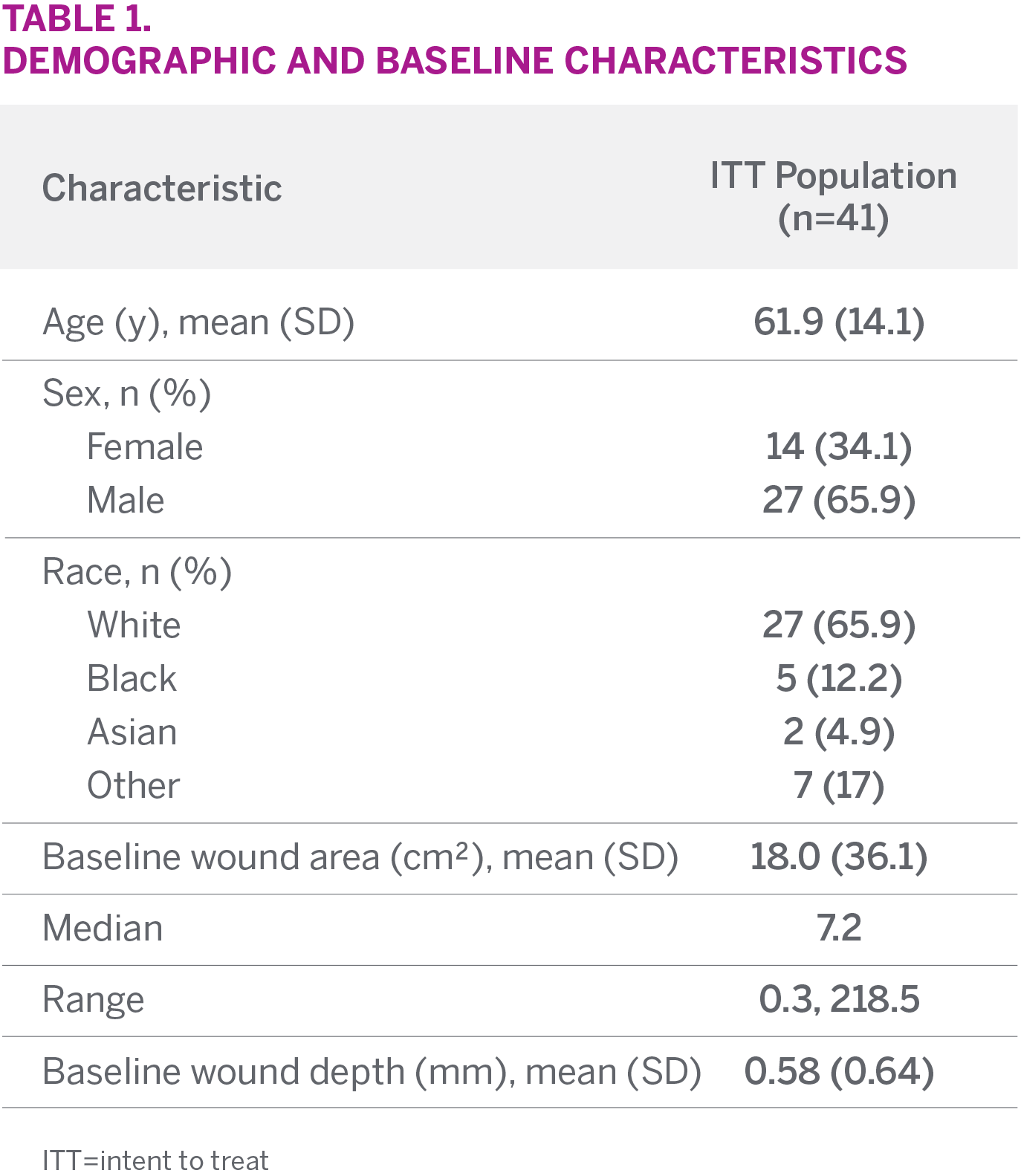
STUDY SUBJECTS
- A total of 41 wounds were included in the ITT analysis
- Wound types included were pressure ulcers (18), surgical wounds (9), VLUs (5), DFUs (4), and other (5)
- Median baseline wound area was 7.2 cm2 and mean depth was 0.58 mm
- Mean wound duration was 103.1 weeks
CHARACTERISTICS
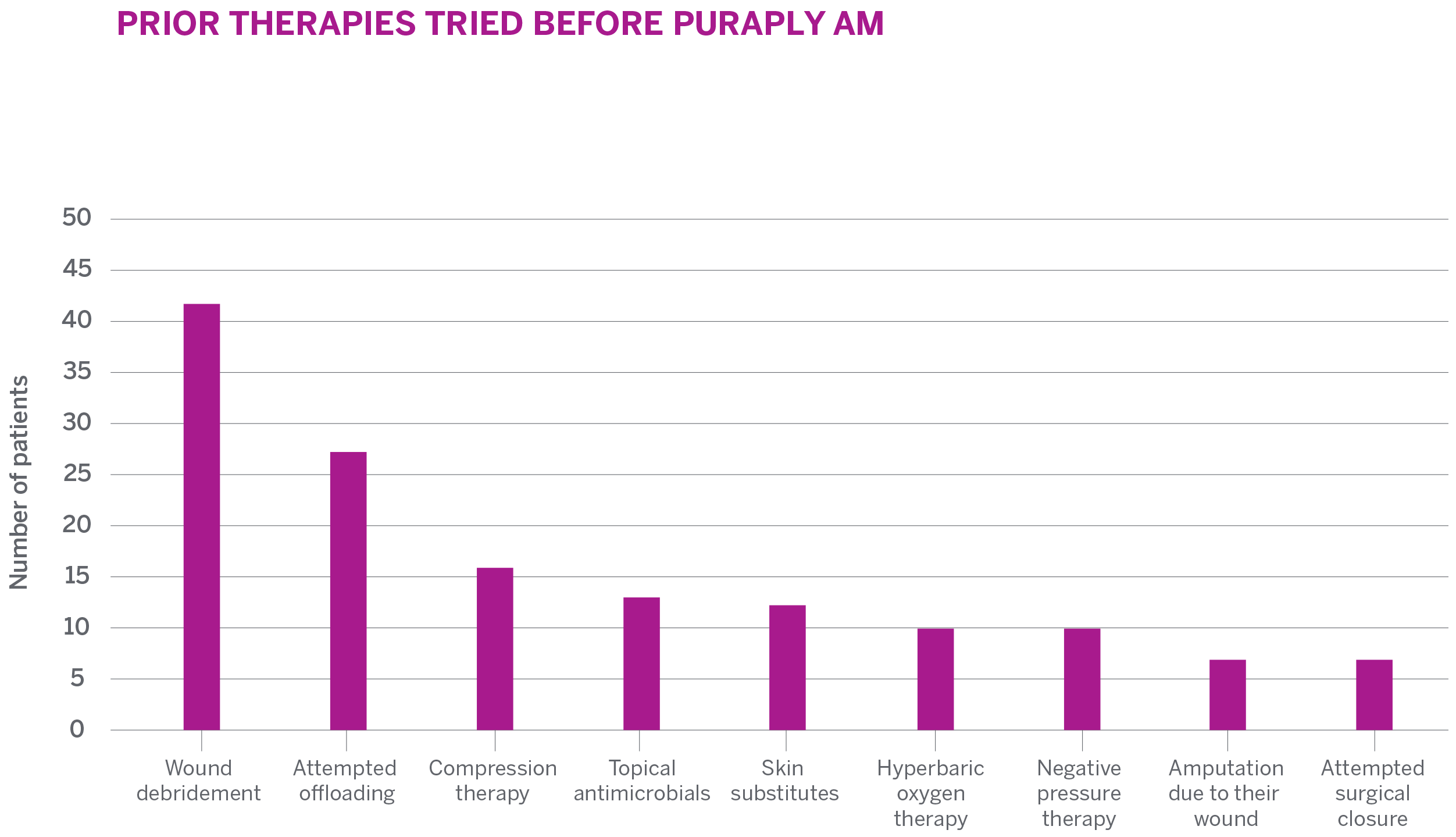
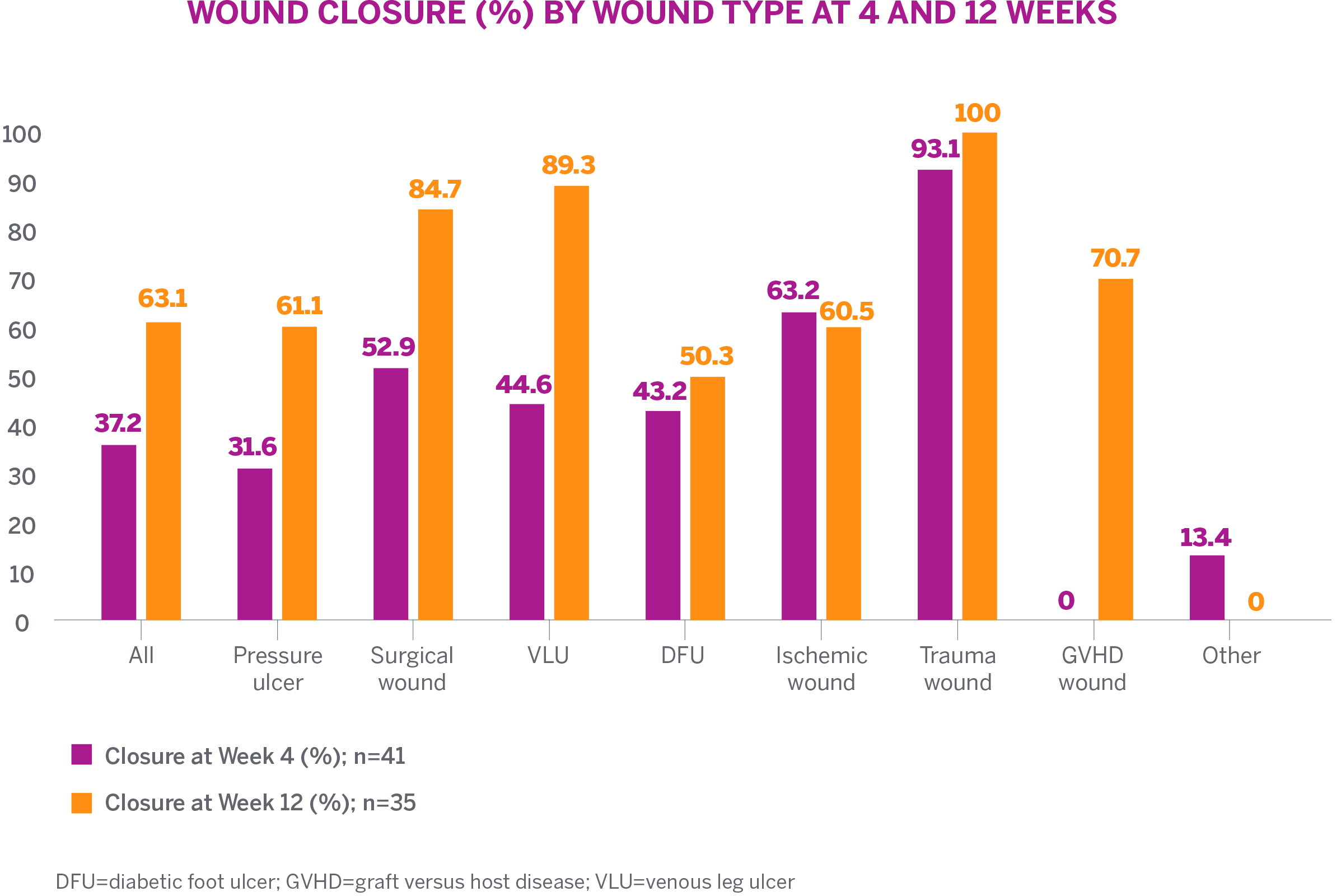
RESULTS
- 73.2% (30/41) demonstrated an overall reduction in wound area over 12 weeks
- 36.6% (15/41) achieved complete wound closure with a mean time to closure of 6.7 weeks
- Increased granulation tissue was observed with healing of wounds
- Mean decrease in wound size of 7.5 cm2
SUPPORT FOR YOU AND YOUR PRACTICE
See how the Organogenesis Circle of Care program can help with billing issues and more, or contact an Organogenesis Tissue Regeneration Specialist to see how PuraPly®AM and PuraPly®XT can help your practice.
Please refer to the PuraPly AM Instructions for Use and PuraPly XT Instructions for Use for complete prescribing information.
REFERENCE:
- Oropallo AR. Plast Reconstr Surg Glob Open. 2019;7:e2047.