PURAPLY®AM & PURAPLY®XT SCIENTIFIC DATA
Discover the antimicrobial effectiveness within PuraPly®AM and PuraPly®XT in nonclinical studies.
ANTIMICROBIAL EFFECTIVENESS AND LOW CYTOTOXICITY
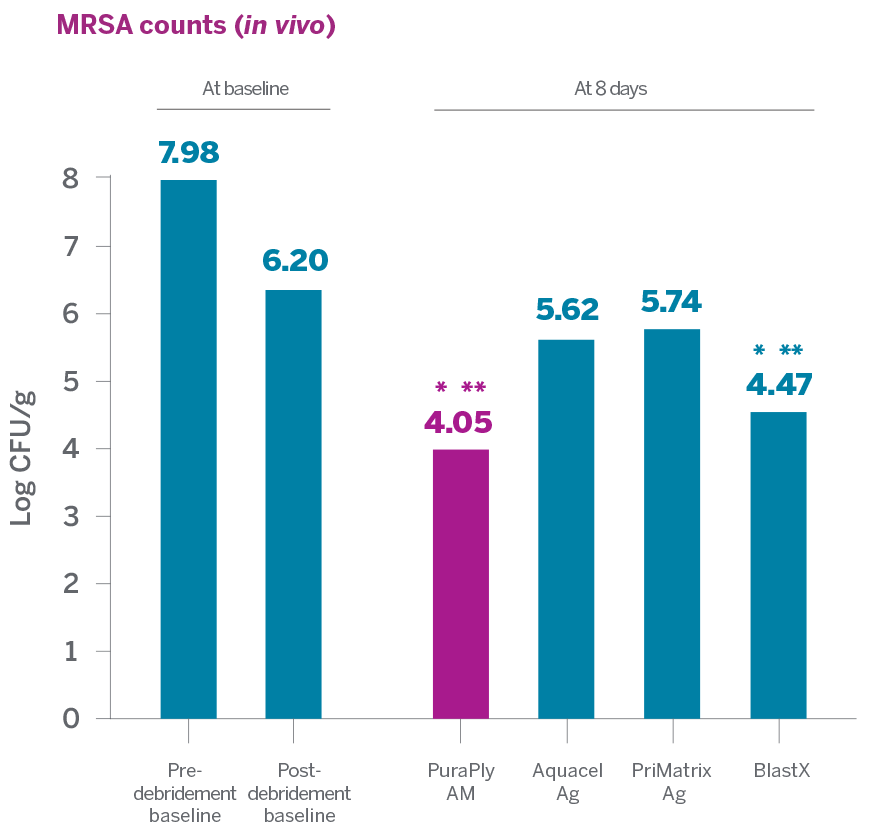
In an in vitro and in vivo nonclinical study to evaluate the antimicrobial capabilities of PuraPly®AM and PuraPly®XT and several other antimicrobial and collagen products against MRSA USA300:
- PuraPly®AM and PuraPly®XT demonstrated the highest antimicrobial activity against MRSA compared to Aquacel Ag and PriMatrix Ag
- PuraPly®AM and PuraPly®XT were able to substantially reduce MRSA in vivo without impairing the wound healing process2
- PuraPly®AM and PuraPly®XT were noncytotoxic, with no detrimental effects in vitro on fibroblast proliferation and viability2
> 99%
PuraPly AM MRSA reduction from
post-debridement baseline1
94.41%
fibroblast viability
at 48 hours for PuraPly AM
BROAD-SPECTRUM ANTIMICROBIAL EFFECTIVENESS
In a United States Pharmacopeia Antimicrobial Effectiveness Test, PuraPly AM effectively reduced concentrations of the following microbes at days 7, 14, and 282:
SUPPORTING WOUND CLOSURE IN REAL-WORLD STUDIES
See how PuraPly AM supported healing in real-world studies, or contact an Organogenesis Tissue Regeneration Specialist to see how PuraPly®AM and PuraPly®XT can help your practice.
Please refer to the PuraPly AM Instructions for Use and PuraPly XT Instructions for Use for complete prescribing information.
REFERENCES:
- Davis SC, et al. Int Wound J. 2021 May 6. doi:10.1111/iwj.13600. Epub ahead of print. PMID: 33955663.
- Data on file. USP Antimicrobial Effectiveness Test. Organogenesis Inc.