WOUNDS COME IN ALL SHAPES AND SIZES
So does PuraPly®AM & PuraPly®XT
WOUND TYPES
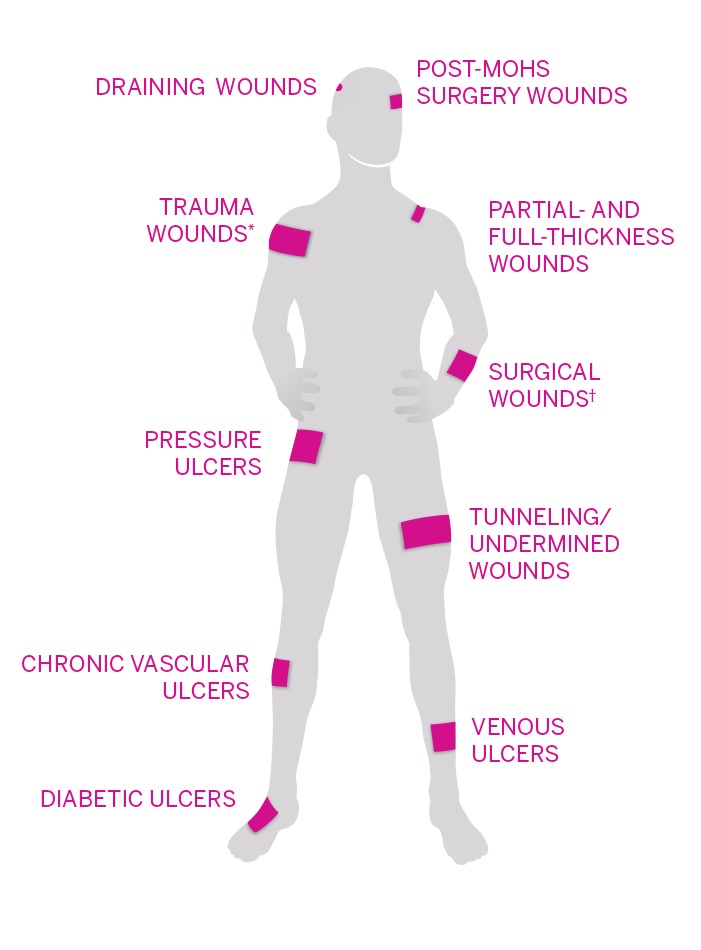
- Draining wounds
- Post-Mohs surgery wounds
- Trauma wounds*
- Partial- and full-thickness wounds
- Pressure ulcers
- Surgical wounds†
- Tunneling/undermined wounds
- Chronic vascular ulcers
- Venous ulcers
- Diabetic ulcers
*Abrasions, lacerations, second-degree burns, skin tears. †Donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence.
APPLYING PURAPLY®AM AND PURAPLY®XT
- Prepare wound to ensure it is free of debris and necrotic tissue
- Cut the dry sheet to the appropriate size and place in contact with wound bed
- Hydrate with sterile saline, do not soak
- Anchor PuraPly AM or PuraPly XT using preferred fixation method such as sutures, adhesive strips, or dermal adhesive and thoroughly cover with a non-adherent dressing
- Cover and wrap with secondary dressings. Reassess at next patient treatment visit. PuraPly AM or PuraPly XT will naturally be resorbed into the wound and is not intended to be removed. Subsequent application is at the discretion of the health care practitioner.
AVAILABLE SIZES + CONFIGURATIONS
- Available in a range of sizes and configurations to manage a wide variety of wound types1
- Appropriate for all sites of care—including physician offices, outpatient departments, and wound care clinics
- Supplied in individually sealed, dry sheets and packaged in sterile, sealed single pouches1
- Can be stored at room temperature1
- Simple application process following sharp debridement, using proper sizing, hydration, fixation, and dressing1
| Product Number | PuraPly AM Product Description | Total Size (cm2) | Billable Units | HCPCS Code | UDI |
|---|---|---|---|---|---|
| 515-030 | PURAPLYAM-COM 1.4 DISC | 2 | 2 | Q4196 | 00618474000183 |
| 515-032 | PURAPLYAM-COM 1.6 DISC | 2 | 2 | Q4196 | 00618474000190 |
| 515-014 | PURAPLYAM-COM 2X2 | 4 | 4 | Q4196 | 00618474000084 |
| 515-050 | PURAPLYAM-COM 2.05X3.05 | 7 | 7 | Q4196 | 00618474000329 |
| 515-016 | PURAPLYAM-COM 2X4 | 8 | 8 | Q4196 | 00618474000091 |
| 515-044 | PURAPLYAM-COM 3.02X3.02 | 10 | 10 | Q4196 | 00618474000299 |
| 515-065 | PURAPLYAM-COM 3X4 | 12 | 12 | Q4196 | 00618474000435 |
| 515-046 | PURAPLYAM-COM 3.76X3.76 | 15 | 15 | Q4196 | 00618474000305 |
| 515-048 | PURAPLYAM-COM 4X4 | 16 | 16 | Q4196 | 00618474000312 |
| 515-008 | PURAPLYAM-COM 5X5 | 25 | 25 | Q4196 | 00618474000107 |
| 515-018 | PURAPLYAM-COM 6X9 | 54 | 54 | Q4196 | 00618474000114 |
| 515-067 | PURAPLYAM-COM 3X4 EXTRA FENESTRATED | 12 | 12 | Q4196 | 00618474000442 |
| 515-069 | PURAPLYAM-COM 4X4 EXTRA FENESTRATED | 16 | 16 | Q4196 | 00618474000459 |
| Product Number | PuraPly XT Product Description | Total Size (cm2) | Billable Units | HCPCS Code | UDI |
|---|---|---|---|---|---|
| 515-071 | PURAPLYAM XT-COM 4.91X4.91 EXTRA FENESTRATED | 24.10 | 25 | Q4197 | 00618474000466 |
REACH OUT TO AN ORGANOGENESIS TISSUE REGENERATION SPECIALIST FOR MORE INFORMATION ON SIZES AND ORDERING.
CONFIRMATION OF CONTROL
Discover the clinical and real-world evidence supporting PuraPly®AM and PuraPly®XT, or contact an Organogenesis Tissue Regeneration Specialist to see how PuraPly AM can help your practice.
Please refer to the PuraPly AM Instructions for Use and PuraPly XT Instructions for Use for complete prescribing information.
REFERENCE:
- PuraPly Antimicrobial [package insert]. Canton, MA: Organogenesis Inc; 2020.