RESPOND REGISTRY
THE FIRST PROSPECTIVE, LARGE, REAL-WORLD EFFECTIVENESS STUDY FOR PURAPLY® AM
The Real-World Effectiveness Study of PuraPly AM on Wounds (RESPOND) was the first prospective, large (N=307), multicenter (28 sites) cohort study to assess the effectiveness of PuraPly AM in various difficult-to-heal wounds.
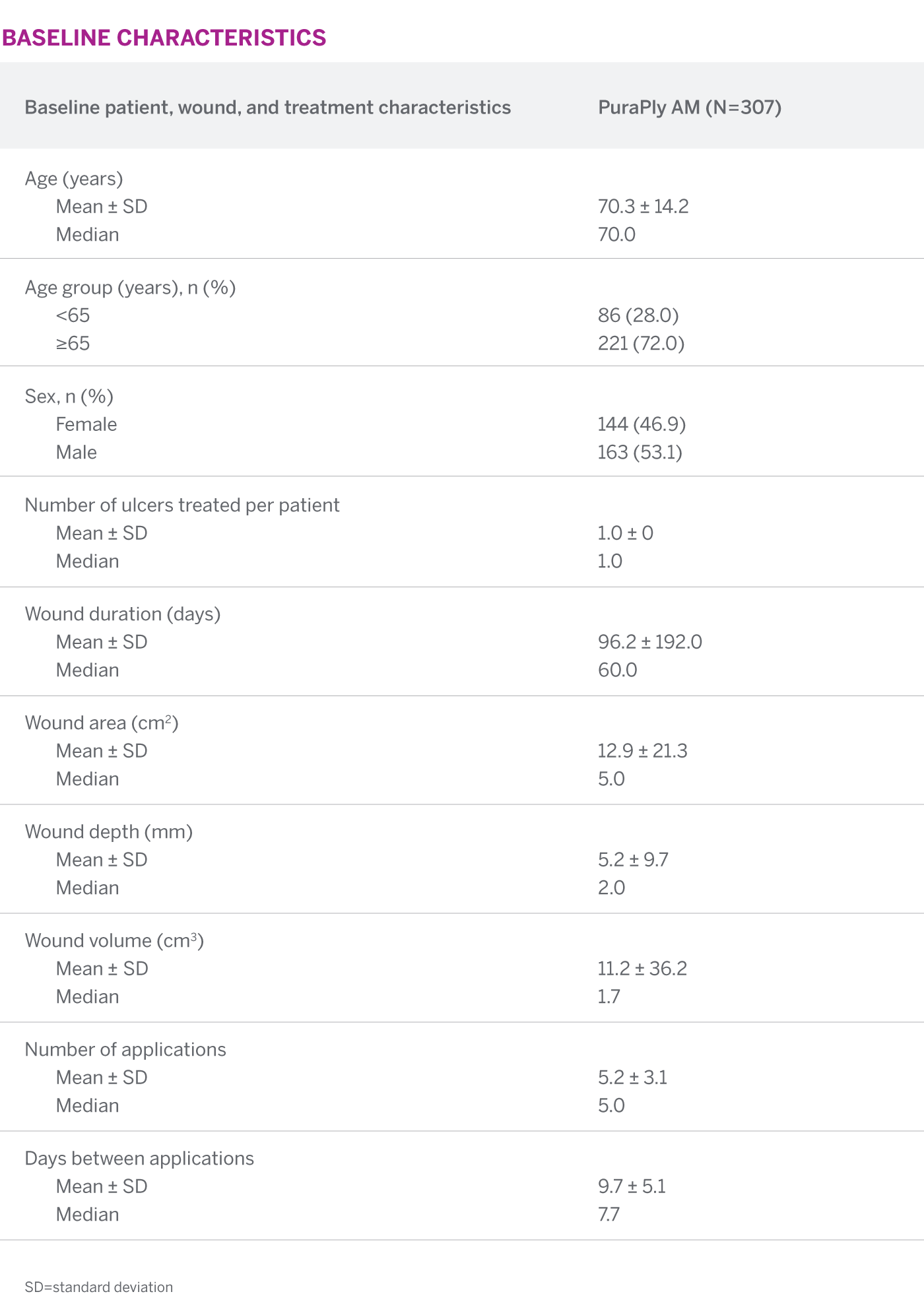
In a primarily elderly population with large, deep, refractory wounds of long durations, PuraPly AM showed that 86% of all PuraPly AM-treated wounds demonstrated improvement in the wound bed condition. Also, 41.5% of wounds achieved complete closure at 12 weeks.1
STUDY BACKGROUND
Large, prospective, noninterventional cohort study to assess the effectiveness of PuraPly AM in real-world clinical settings at 28 sites.
The primary endpoints were frequency and time to wound closure, and secondary endpoints were percent reduction in wound area, depth, and volume. Other endpoints included improvement in wound bed condition.
- Postmarketing, prospective, noninterventional, multicenter cohort study (NCT03286452)
- Patients received standard of care in addition to PuraPly AM
- Wounds were cleansed and debrided at initial visit
- At each study visit, digital photography was used to image the wounds, and data was collected for wound measurements (length, width, and depth)
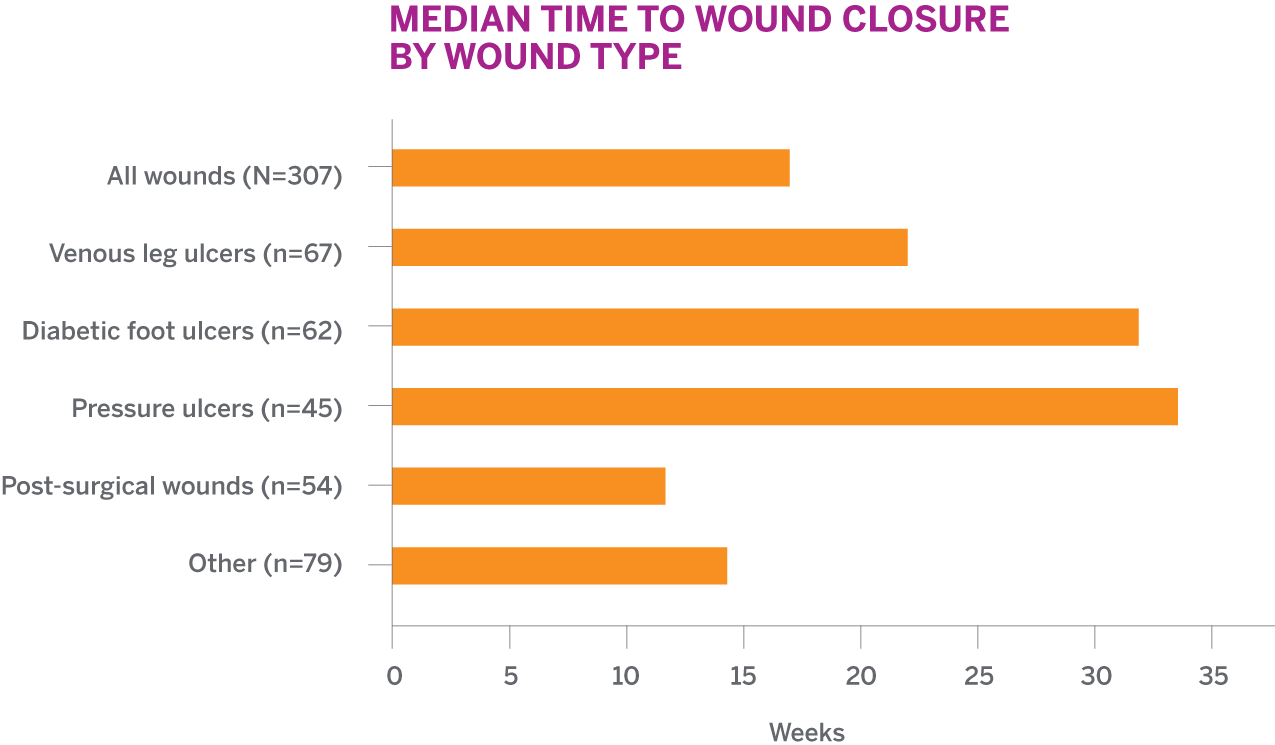
- N=307 (67 VLUs, 62 DFUs, 45 pressure ulcers, 54 post-surgical wounds, and 79 other wounds)
- Mean wound duration at baseline: 96.2 days
- Mean wound area at baseline: 12.9 cm2
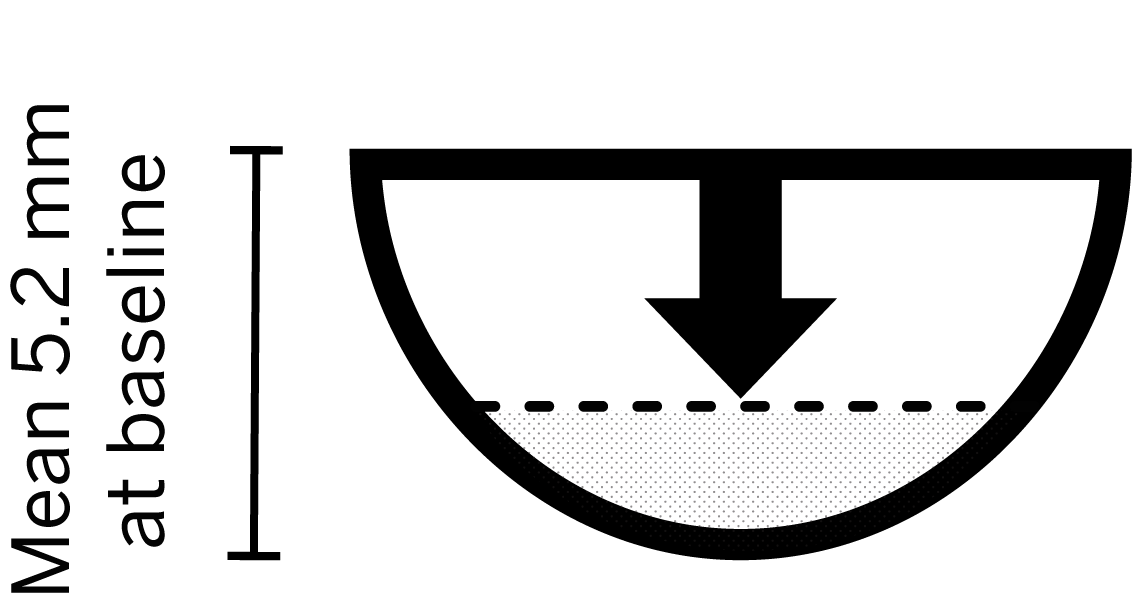
- Mean wound depth at baseline: 5.2 mm
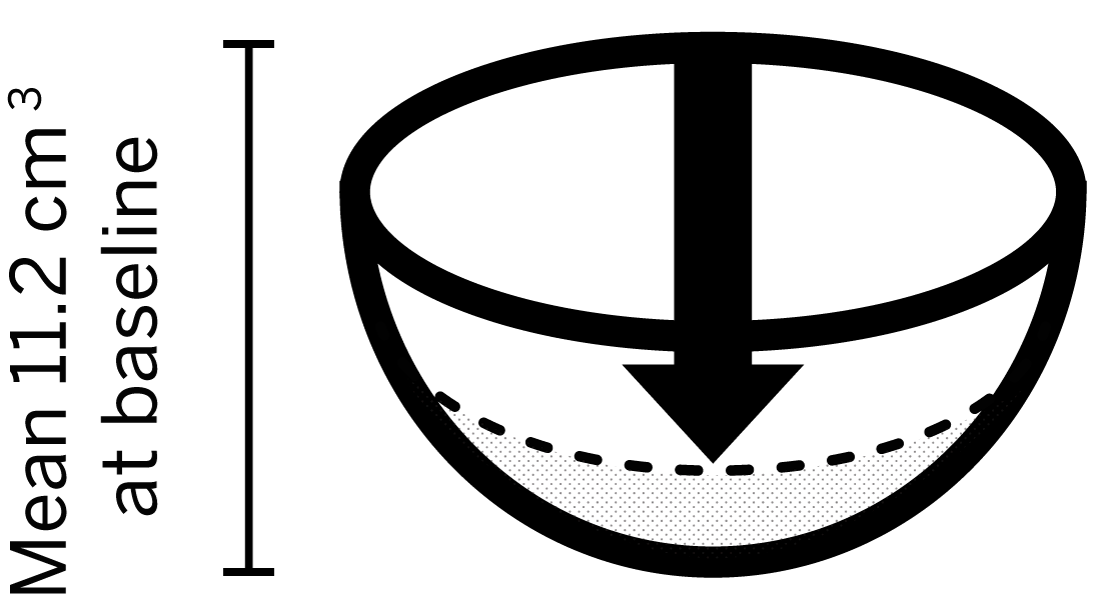
- Mean wound volume at baseline: 11.2 cm3
- Mean number of applications: 5.2; 21.8% of patients received 1 to 2 applications
RESULTS
PuraPly AM supported healing in large,* difficult-to-heal wounds
*12.9 cm2 mean wound area at baseline86%
of all PuraPly AM-treated wounds demonstrated improvement in the wound bed condition:
INCREASEDHEALTHYGRANULATIONTISSUE
REDUCEDEXUDATE

READINESSFOR OTHERADVANCED SKINSUBSTITUTES
41.5%
CLOSURE RATEAT 12 WEEKSFOR ALL WOUNDS
17 WEEKS
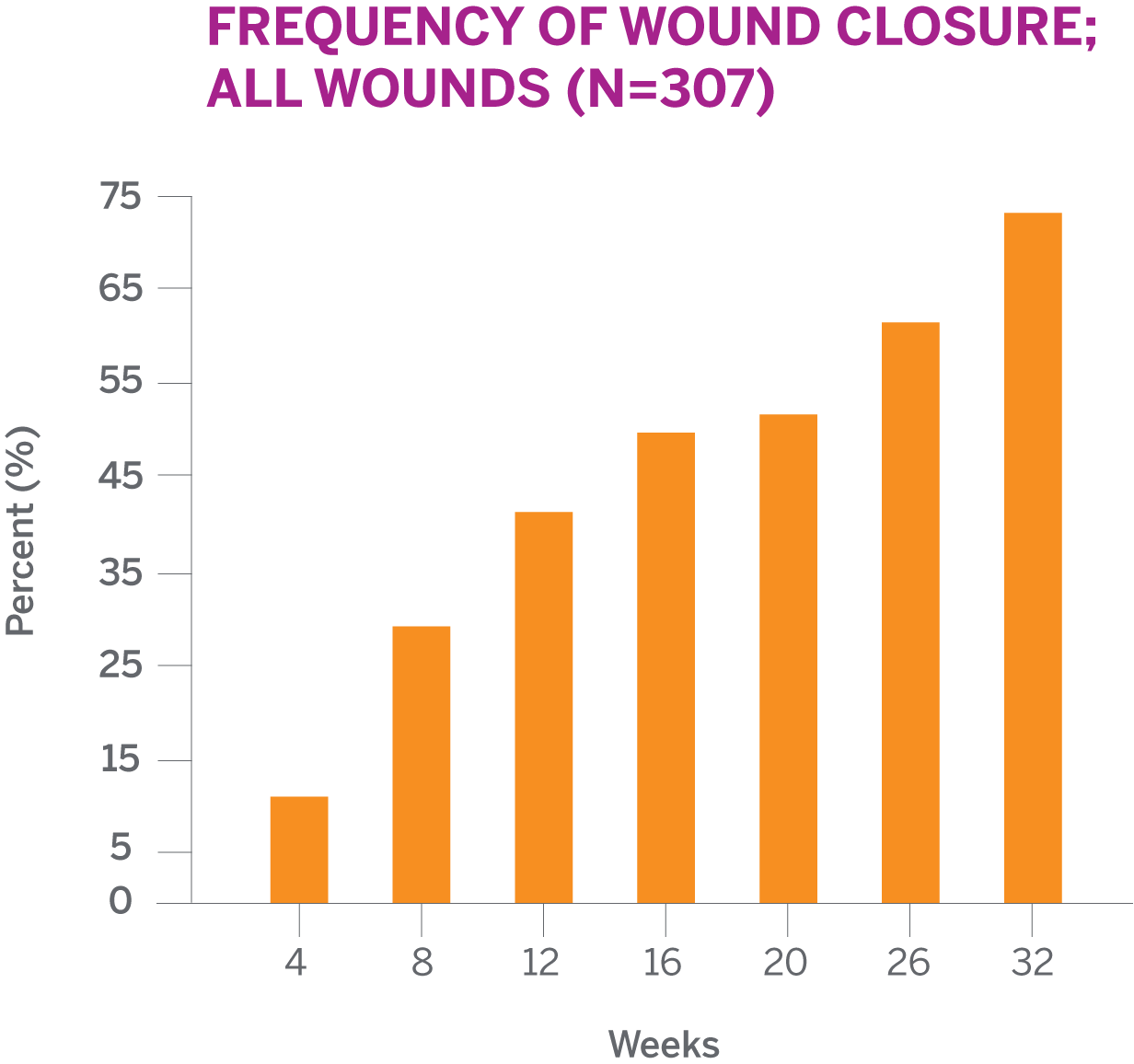
MEDIAN TIMETO CLOSUREFOR ALL WOUNDS
41.5% of PuraPly AM-treated wounds achieved complete wound closure at 12 weeks
SEE FREQUENCY OF WOUND CLOSURE BY WEEKThe median time to wound closure for all PuraPly AM-treated wounds was 17 weeks
SEE MEDIAN TIME TO WOUND CLOSURE BY TYPEFor all 307 patients treated with PuraPly AM
81%
of wounds achieved >60% reduction in area*

71%
of wounds achieved >60% reduction in depth*
85%
of wounds achieved >75% reduction in volume*
SUPPORTING HEALING IN STALLED WOUNDS
See how PuraPly AM supported healing in chronic wounds that failed ~2 years of prior therapies, or contact an Organogenesis Tissue Regeneration Specialist to see how PuraPly AM can help your practice.
Please refer to the PuraPly AM Instructions for Use and PuraPly XT Instructions for Use for complete prescribing information.
REFERENCE:
- Bain MA, et al. J Comp Eff Res. 2020:9(10)691-703. doi:10.2217/cer-2020-0058